Breaking local symmetry—why water freezes but silica forms a glass
4.5 (415) · $ 8.00 · In stock
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.

Glass transition - Wikipedia
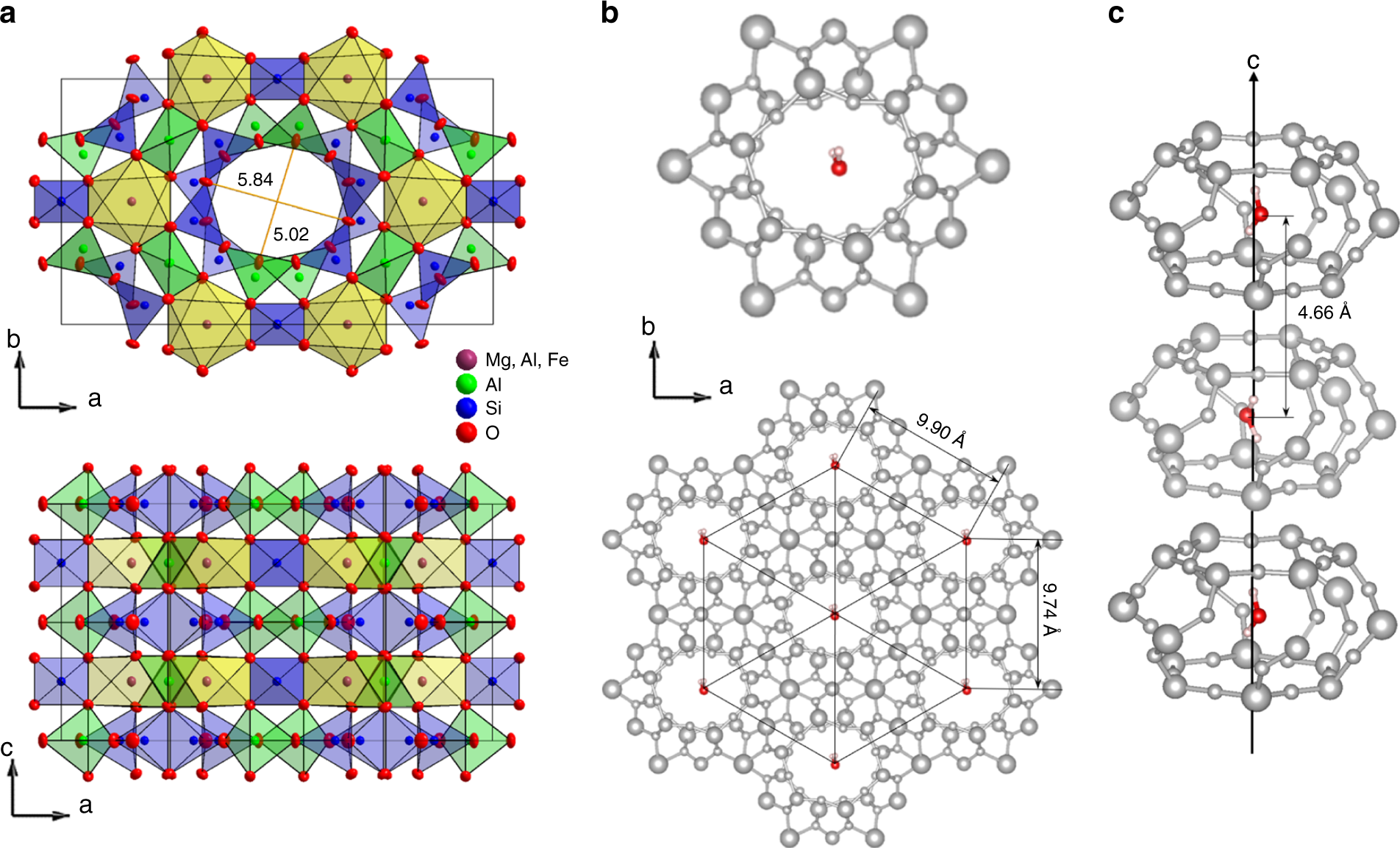
Dielectric ordering of water molecules arranged in a dipolar lattice

Coatings, Free Full-Text
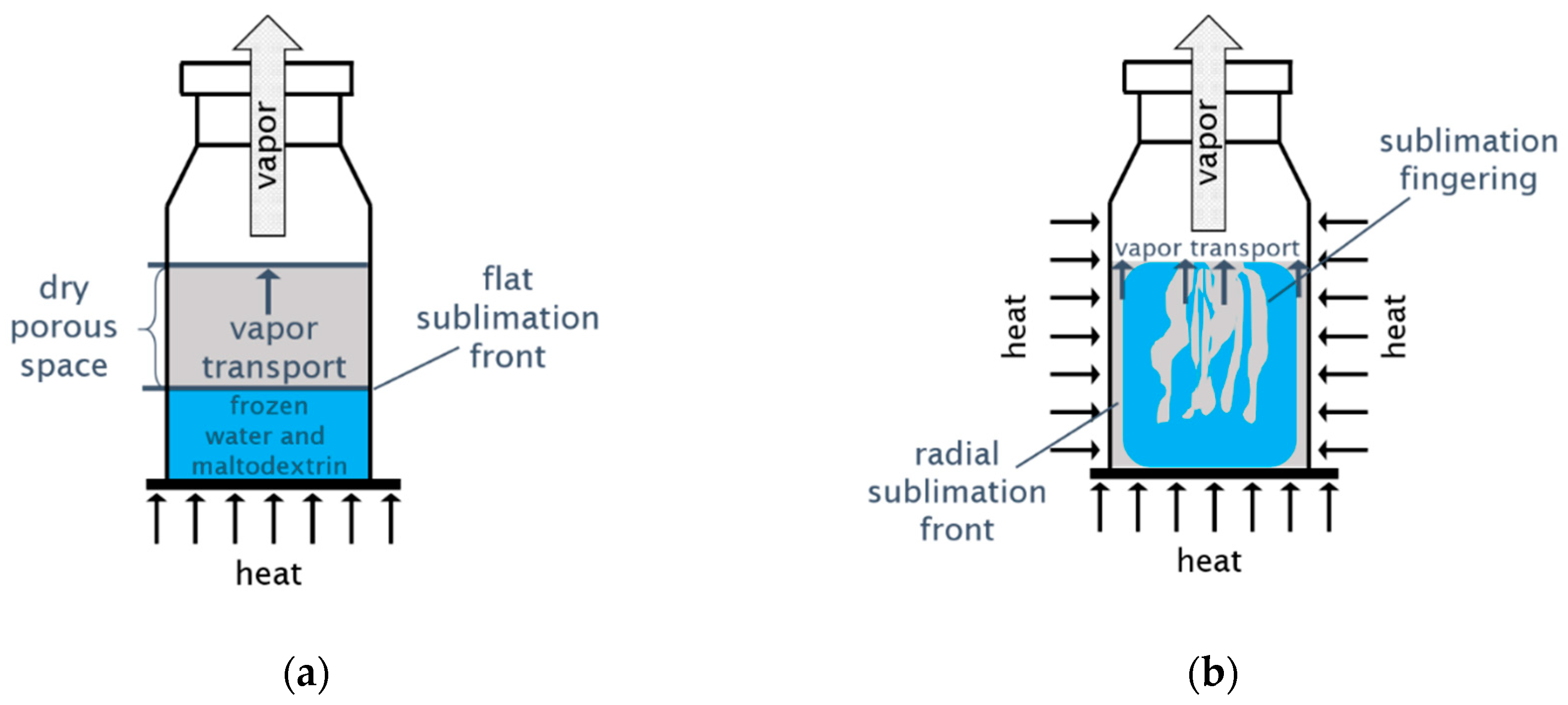
Processes, Free Full-Text
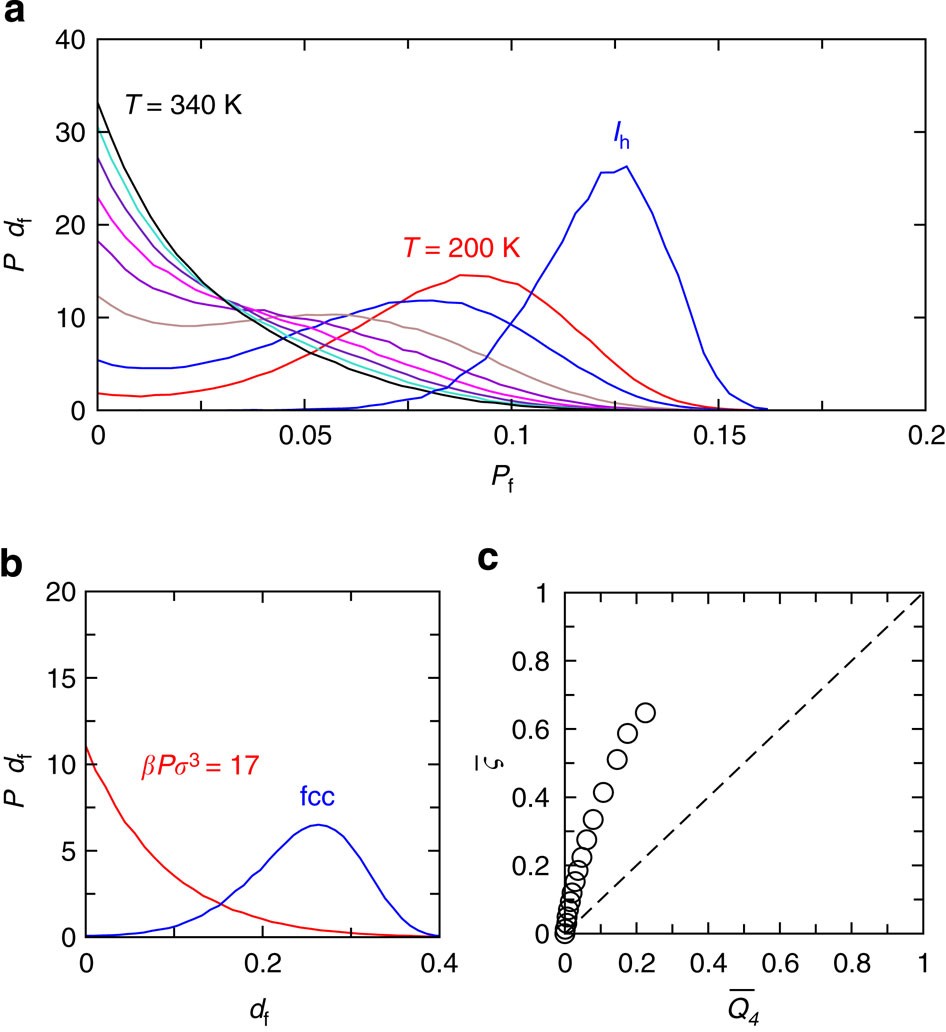
Understanding water's anomalies with locally favoured structures

Critical cooling rate versus reduced glass transition temperature T rg

Structure and dynamics of nanoconfined water and aqueous solutions
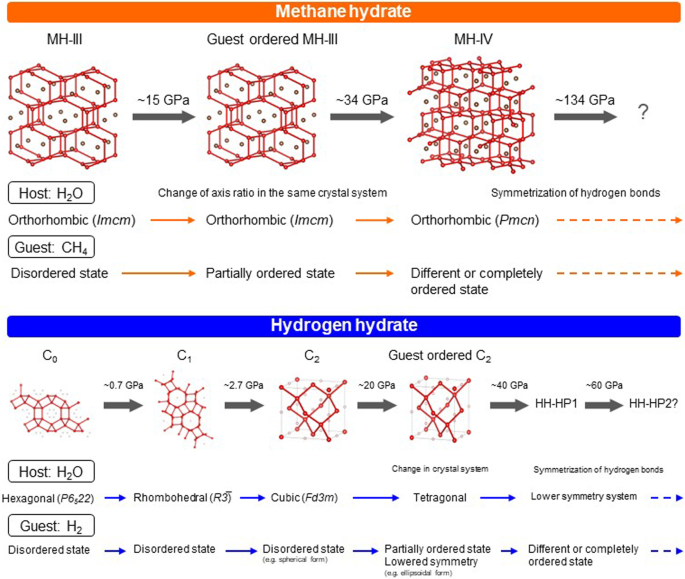
Significance of the high-pressure properties and structural evolution of gas hydrates for inferring the interior of icy bodies, Progress in Earth and Planetary Science
Dehydration of a crystal hydrate at subglacial temperatures

Catalysts, Free Full-Text










